Home Use Covid-19 Antigen Rapid Test Strip Cassette Swab Kit
Fast: 15 Minutes;
Cheap: Lower cost with high efficiency;
Simple: Easy sample collection;
Convenient: Can be home use;
INTENDED USE OF ANTIGEN TEST
The product is used for the qualitative detection of SARS-COV-2 infection. The entire detection process takes only 15-20 minutes, and the operation is simple and sensitive. No instrument required. It can be used for the screening of early infected patients and asymptomatic patients. This method is an effective supplement for nucleic acid detection.
PRINCIPLE OF ANTIGEN TEST
The detection of SARS-COV-2 adopts the principle of double antibody sandwich method and colloidal gold immunochromatography to qualitatively detect SARS-COV-2 antigen in human Nasal swabs, pharyngeal swabs, sputum, bronchoalveolar lavage fluid, etc., with two highly specific and highly sensitive SARS-COV-2 N antigen monoclonal antibodies, wherein monoclonal antibody I is a capture antibody, fixed in the detection area on the NC membrane, monoclonal antibody II is a colloidal gold-labeled antibody, sprayed on the binding pad, and the NC membrane quality control area C is coated with goat anti-mouse IgG antibody and goat anti-rabbit IgG antibody . The double antibody sandwich method is used in the detection area, and the antigen-antibody reaction is used in the quality control area, combined with colloidal gold immunochromatography technology to detect the SARS-COV-2 in the human body. During detection, the sample is chromatographed under the capillary effect. If the tested sample contains SARS-COV-2, the gold-labeled SARS-COV-2 N antigen monoclonal antibody I combines with SARS-COV-2 to form a complex, and combines with the a SARS-COV-2 N antigen monoclonal antibody II fixed at the detection line during the chromatography process, which will form the "Au-antibody I-N antigen- antibody II" sandwich, so that a purple band appears in the detection area (T); Otherwise, no magenta bands appear in the detection area (T). Regardless of whether there is a SARS-COV-2 antigen in the sample, the complex will continue to be chromatographed up to the control area (C), and a purple band appears when reacting with the goat anti-mouse IgG antibody and goat anti-rabbit IgG antibody.
The purple-red band presented in the control area (C) is a standard for judging whether the chromatographic process is normal, and also serves as an internal control standard for reagents.
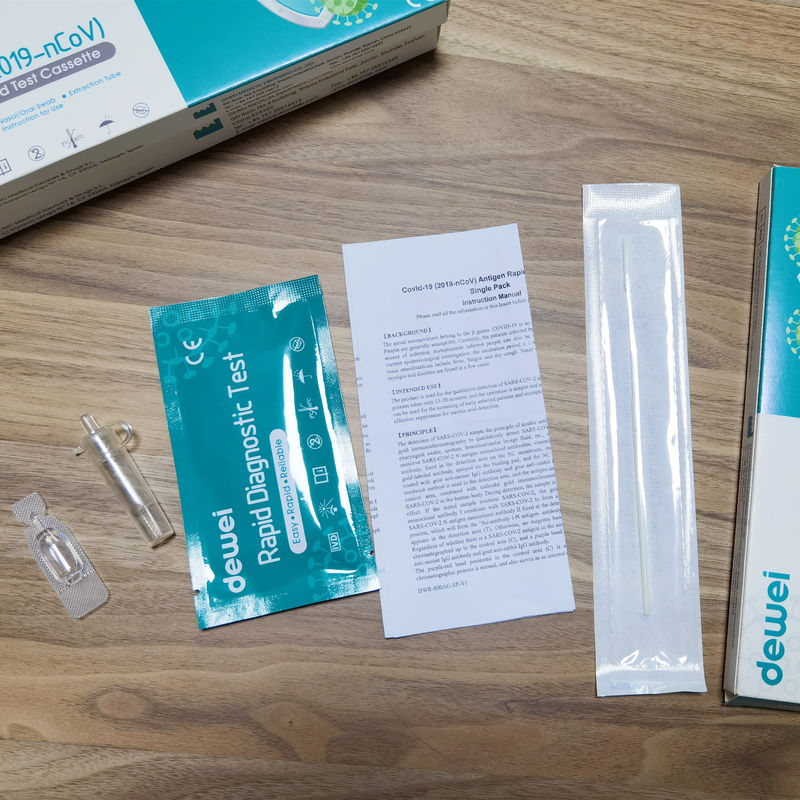
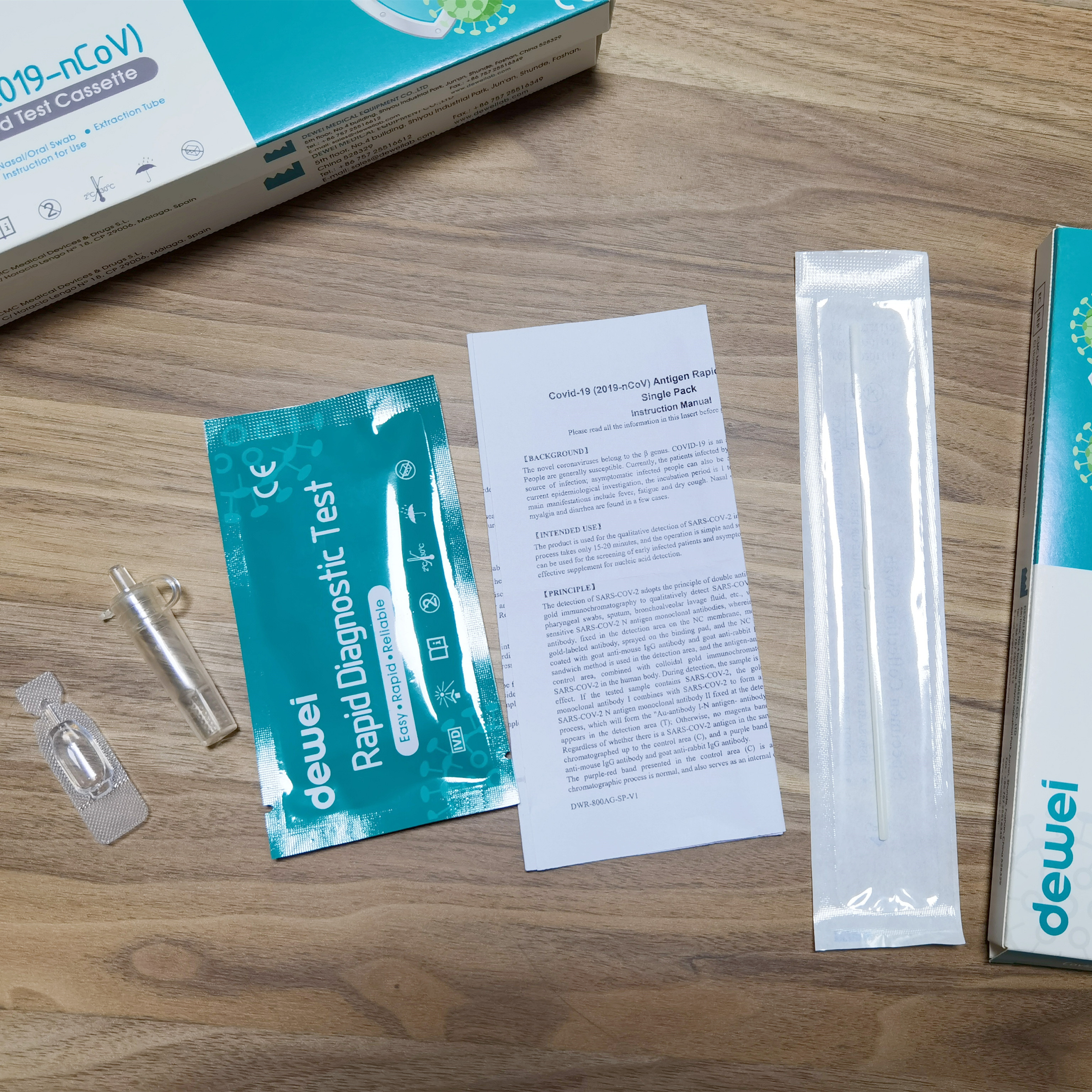
MAIN CONTENTS OF ANTIGEN TEST
• One pouch containing a rapid test Cassette with desiccant.
• Extraction tube.
• Extraction Reagent.
• Instructions for use.
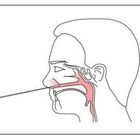
• Nasal/Oral Swab.
STORAGE AND STABILITY
• Store at 39~ 86 º F (4 ~ 30 º C) in the sealed pouch for 18 months.
PRECAUTIONS
• For in vitro diagnostic use only.
• Do not use after expiration date.
• The test Cassette should remain in the sealed pouch until use.
• The used test Cassette should be discarded according to local regulations.
LIMITATIONS
• The etiology of respiratory infection caused by microorganisms other than SARS-COV-2 will not be established with this test. The Coronavirus Ag Rapid Test Cassette (Swab) is capable of detecting both viable and non-viable SARS-COV-2. The performance of the Coronavirus Ag Rapid Test Cassette (Swab) depends on antigen load and may not correlate with viral culture results performed on the same specimen.
• Failure to follow the Test Procedure may adversely affect test performance and/or invalidate the test result.
• If the test result is negative and clinical symptoms persist, additional testing using other clinical methods is recommended. A negative result does not at any time rule out the presence of SARS-CoV-2 antigens in specimen, as they may be present below the minimum detection level of the test or if the sample was collected or transported improperly.
• As with all diagnostic tests, a confirmed diagnosis should only be made by a physician after all clinical and laboratory findings have been evaluated.
• Positive test results do not differentiate between SARS-COV and SARS-COV-2.
• Negative results should be treated as presumptive and confirmed with an FDA authorized molecular assay, if necessary, for clinical management, including infection control.
DIRECTION OF USE





 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!