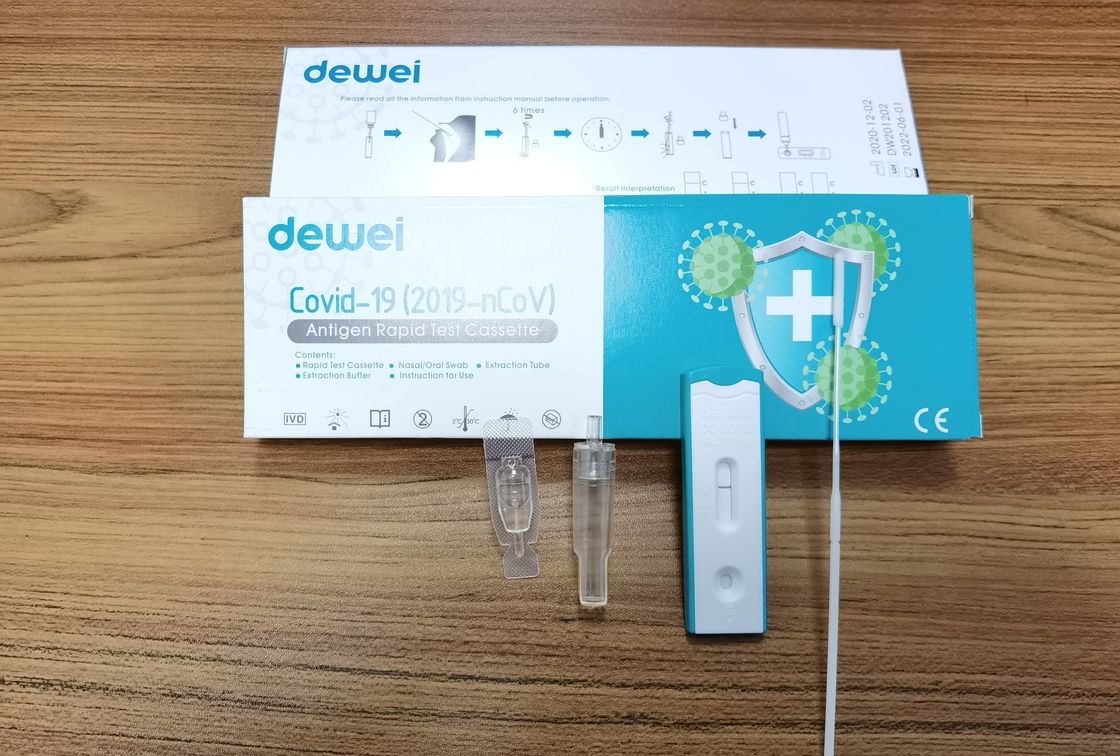
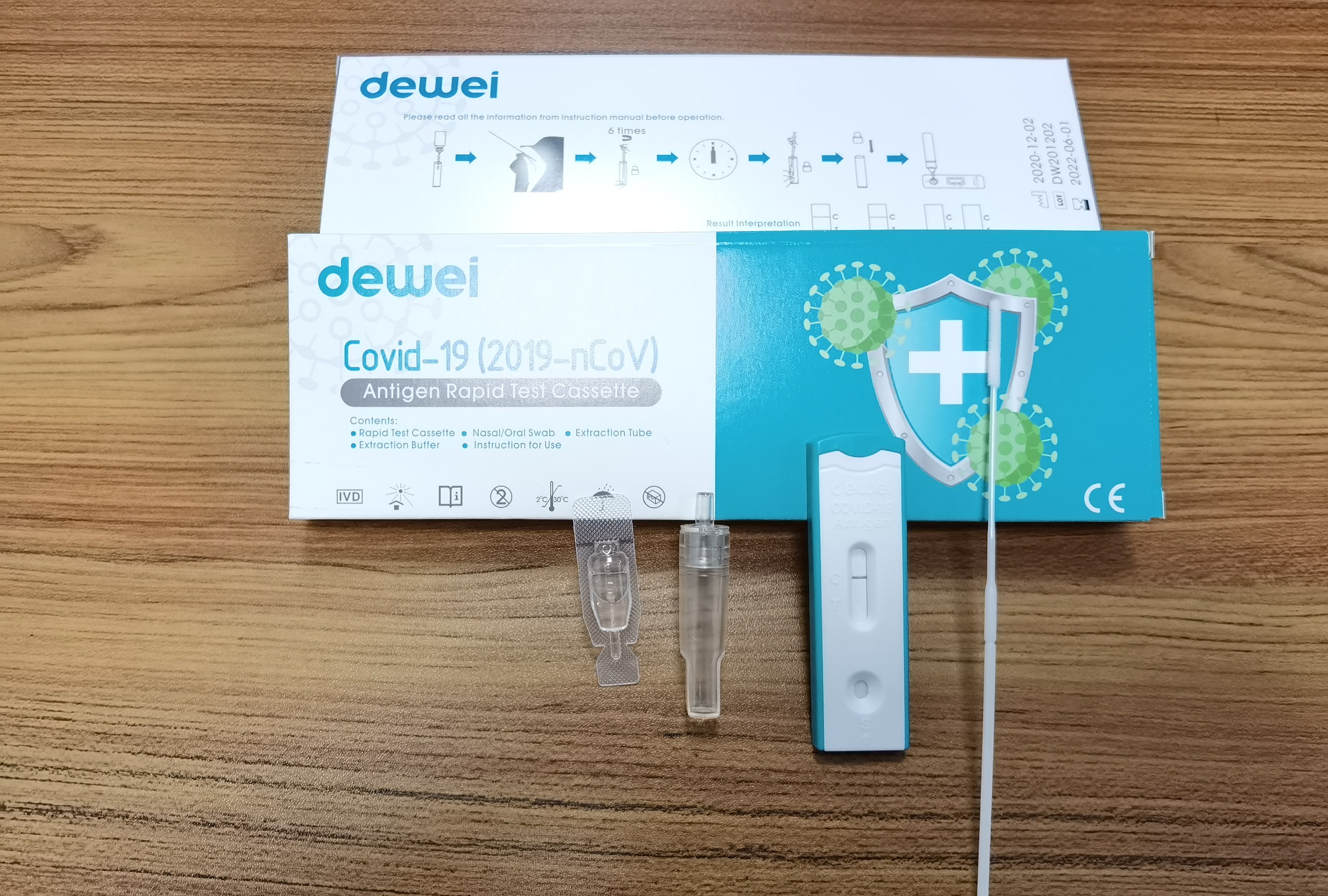
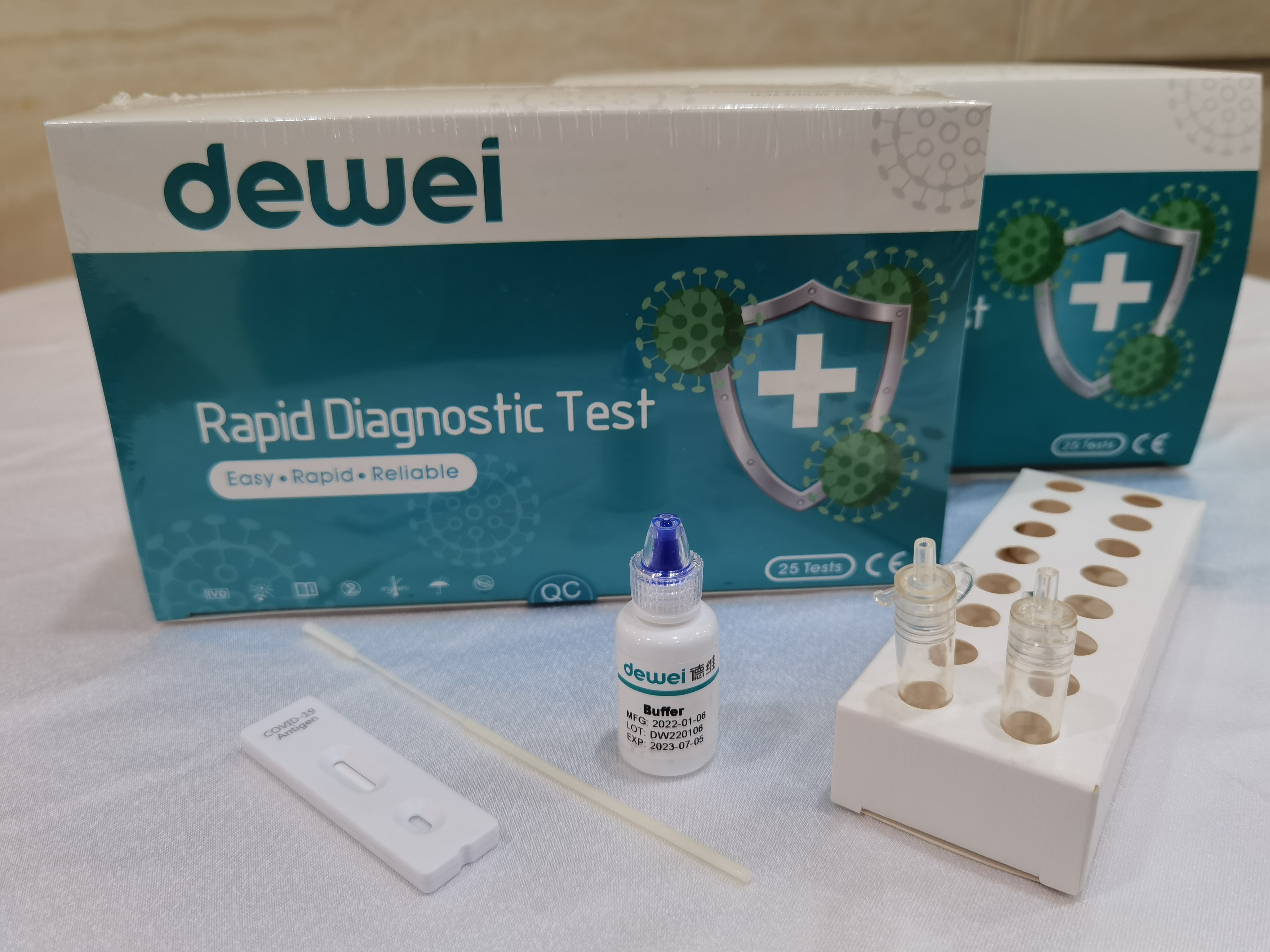
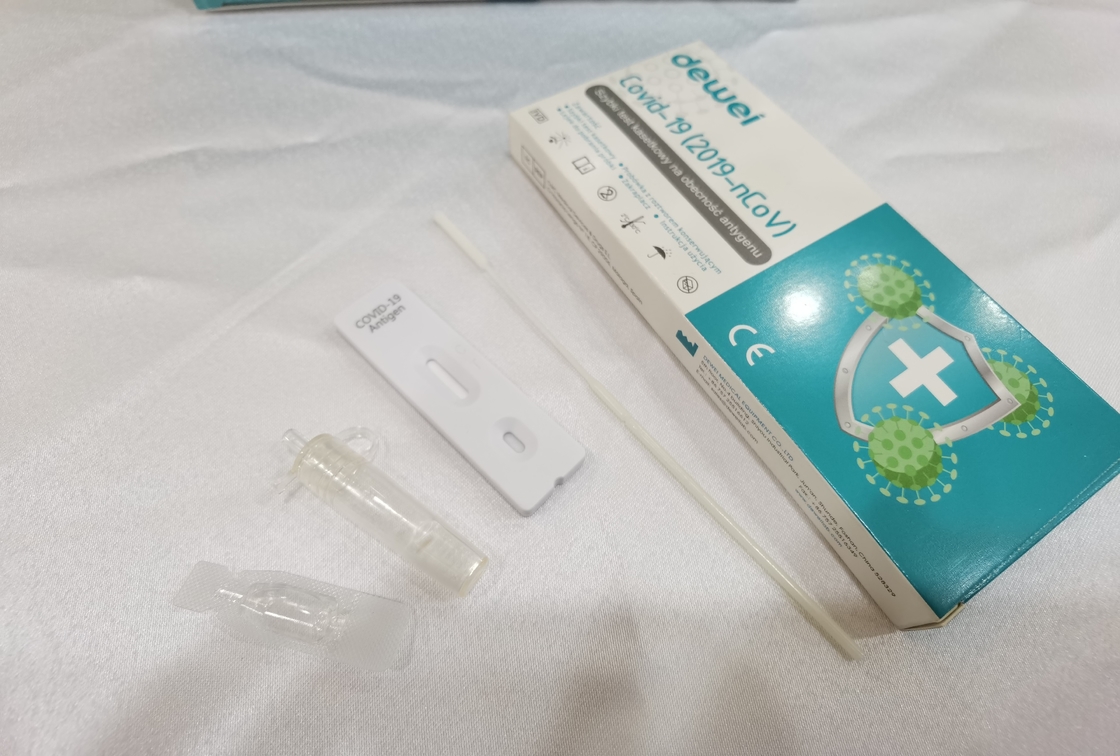
Fast One Step POCT 2019-NCoV Antigen Rapid Test Strip Cassette Kit CE marked
Single Pack Self Test for Home Used, 25tests/box for Professional Used!
Intended use of Covid-19 Antigen Rapid Test
The rapid test kit is used for qualitative determination of novel coronavirus (SARS-CcV-2) antigen in human nasal swab samples in vitro.. This kit is offered to clinical laboratories and healthcare workers for point-of-care testing, and not for at home testing, in compliance with Section IV.D. of the FDA’s Policy for COVID-19 Diagnostic Test.
Principle of Covid-19 Antigen Rapid Test
After an appropriate amount of sample is added to the detection well, the sample moves under the action of the capillary. The new coronavirus antigen in the sample will combine with colloidal gold-labeled new coronavirus N protein antibody to form a colloidal gold-antigen-antibody complex. The immune complex product is then chromatographed along the nitrocellulose membrane to the detection area (T), binds to the pre-coated N protein monoclonal antibody, and forms a purple line, indicating that the new coronavirus antigen is positive.
The quality control antibody-labeled colloidal gold particles are chromatographed to the quality control area (C) and combined with the pre-coated anti-quality control antibody to form a purple C line, indicating that the test is effective. If the QC line does not appear, the test result is invalid.
Applicable Department
Emergency Department
ICU
Pneumology Department
Cardio-Pulmonary Function Department
When should you perform Dewei COVID-19 Ag Rapid Test?
The COVID-19 Ag Rapid test should NOT be used for diagnosis of COVID-19 infection. Any individual who is symptomatic or a contact of a confirmed case should be directed to their healthcare provider, an assessment centre, or participating licensed community lab to seek PCR testing.
Do long-term care homes have to conduct the rapid antigen tests themselves?
The ministry is exploring developing a Vendor of Record to connect LTC homes wishing to contract with providers to conduct surveillance screening clinics on their behalf in the LTC home. The ministry would look to developing and providing the necessary templates and service agreements to support licensees in making decisions regarding entering into contracts with providers and the terms and conditions on which services are provided. The Vendor of Record would be optional and would not prevent homes from contracting with a qualified vendor that is not on this roster.
If an individual has been vaccinated or immunized for COVID-19, do they still need to be tested prior to visiting a long-term care home?
Yes, the testing requirements of the Minister’s Directive continue to apply to individuals who have been vaccinated, in addition to continuing to follow public health measures including masking, physical distancing, hand hygiene, and symptom screening. This includes active screening on entry to the long-term care home for symptoms and exposures for COVID-19, including temperature checks, attesting to not be experiencing any of the typical and atypical symptoms of COVID-19 (in accordance with Directive #3 issued by the Chief Medical Officer of Health).
Do individuals who test positive on the rapid antigen test need to be confirmed with lab-based PCR testing?
A positive test result on the rapid antigen test should be considered a preliminary positive and requires a confirmatory laboratory-based PCR test. The following actions should be taken:
1. Counsel individual that the result is preliminary positive and PCR confirmation is required.
2. Issue guidance to return home and self-isolate until receipt of confirmatory laboratory PCR test result.
3. Ensure confirmatory laboratory-based PCR testing is performed within 24 hours.
4. Report the preliminary positive result to the local Public Health unit as soon as possible.
Is a new specimen required for the confirmatory laboratory-based PCR test when an individual tests positive on the rapid antigen test?
A new specimen is required from the individual that tests positive on the rapid antigen test for the confirmatory laboratory-based PCR test.

 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!