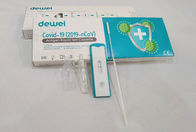
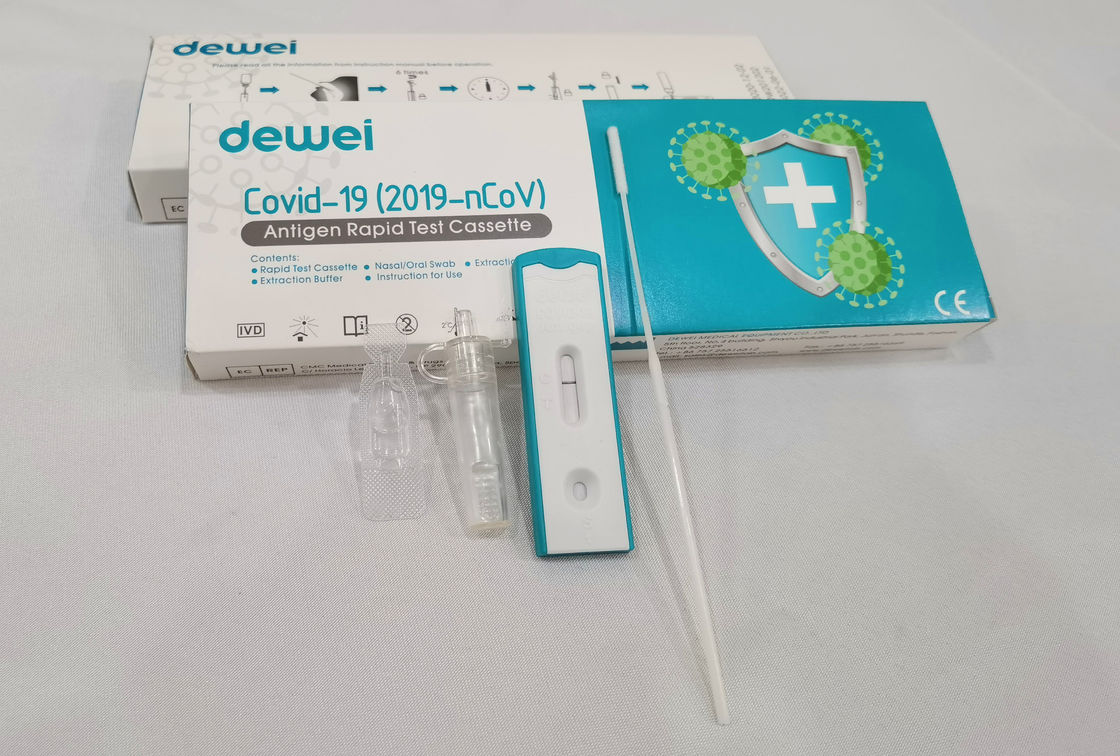
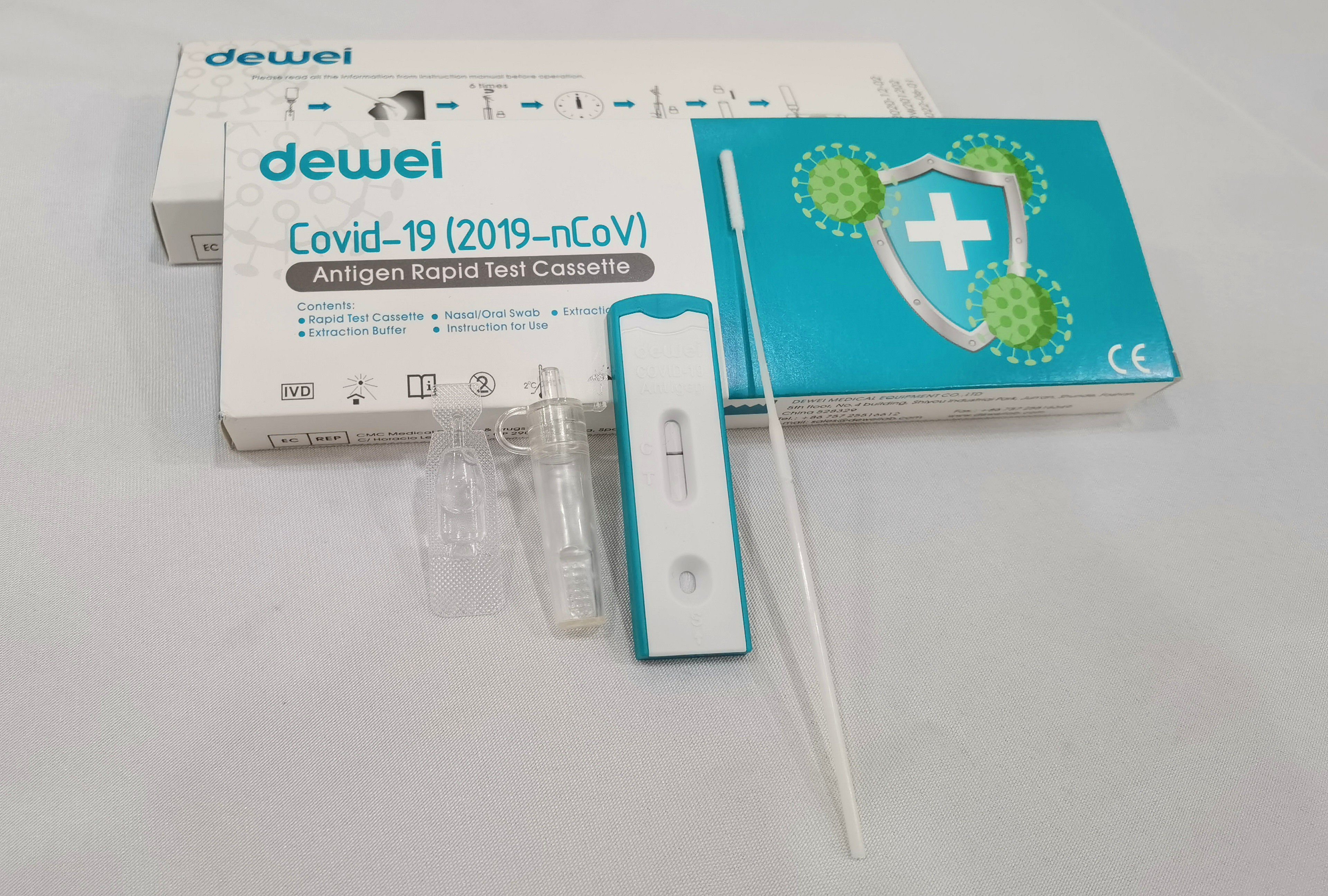
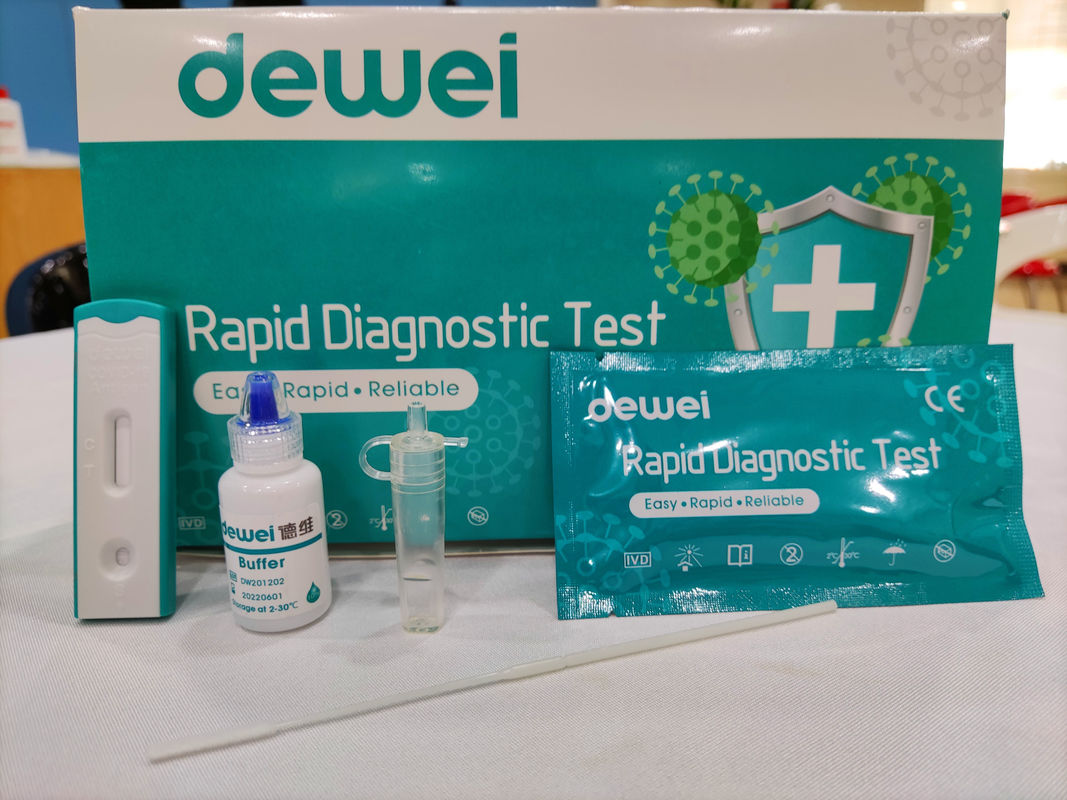
Point of Care Novel Corona Virus POCT 2019-NCoV Rapid Test Swab Antigen Single Pack Self Test Kit
Advantages of Antigen Rapid Test
² Fast: 15 Minutes
² Easy: One Step
² Convenient: Throat/Nasal swab sample
² Cheap: Lower cost with high efficiency
Intended use of SARS-CoV-2 Antigen Test
The rapid test kit is used for qualitative determination of novel coronavirus (SARS-CoV-2) antigen in human nasal swab samples in vitro.. This kit is offered to clinical laboratories and healthcare workers for point-of-care testing, and not for at home testing, in compliance with Section IV.D. of the FDA’s Policy for COVID-19 Diagnostic Test.
Applicable Department of SARS-CoV-2 Antigen Test
Emergency Department
ICU
Pneumology Department
Cardio-Pulmonary Function Department
Sample Collection of SARS-CoV-2 Antigen Test
Ø Nasal / Nasopharyngeal Sampling: Gently insert the swab head into the nasal cavity and scrape the cavity wall. Immerse the swab head into sample solution and discard the tail of swab.
Ø Oral / Oropharyngeal Sampling: Use the swab to wipe the bilateral pharyngeal tonsils and posteriorpharyngeal wall, immerse the swab head into sample solution and discard the tail of swab.
All specimens should be tested as soon as early they are prepared. If necessary, they may be stored at 2-8°C for up to 24 hours or at -20°C for longer periods. Restore the sample to room temperature before test.
Principle of SARS-CoV-2 Antigen Test
After an appropriate amount of sample is added to the detection well, the sample moves under the action of the capillary. The new coronavirus antigen in the sample will combine with colloidal gold-labeled new coronavirus N protein antibody to form a colloidal gold-antigen-antibody complex. The immune complex product is then chromatographed along the nitrocellulose membrane to the detection area (T), binds to the pre-coated N protein monoclonal antibody, and forms a purple line, indicating that the new coronavirus antigen is positive.
The quality control antibody-labeled colloidal gold particles are chromatographed to the quality control area (C) and combined with the pre-coated anti-quality control antibody to form a purple C line, indicating that the test is effective. If the QC line does not appear, the test result is invalid.
Test Result of SARS-CoV-2 Antigen Test




 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!