HCV Hepatitis C Virus Test Device Rapid Test Strip Cassette
INTENDED USE
The HCV Test Device (Serum/Plasma) is a rapid visual immunoassay for the qualitative, presumptive detection of antibodies to HCV in human serum or plasma specimens. This kit is intended for use as an aid in the diagnosis of HCV infection.
INTRODUCTION
Hepatitis C Virus (HCV) is a small, enveloped, positive-sense, single-stranded RNA virus. HCV is now known to be the major cause of parenterally transmitted non-A, non-B hepatitis. Antibodies to HCV are found in over 80% of patients with well-documented non-A, non-B hepatitis. Conventional methods fail to isolate the virus in cell culture or visualize it by electron microscope. Cloning the viral genome has made it possible to develop serologic assays that use recombinant antigens. Compared to first generation HCV EIAs using single recombinant antigens, new serologic tests include multiple antigens using recombinant protein and/or synthetic peptides to avoid nonspecific cross-reactivity and to increase sensitivity.
MAIN CONTENTS
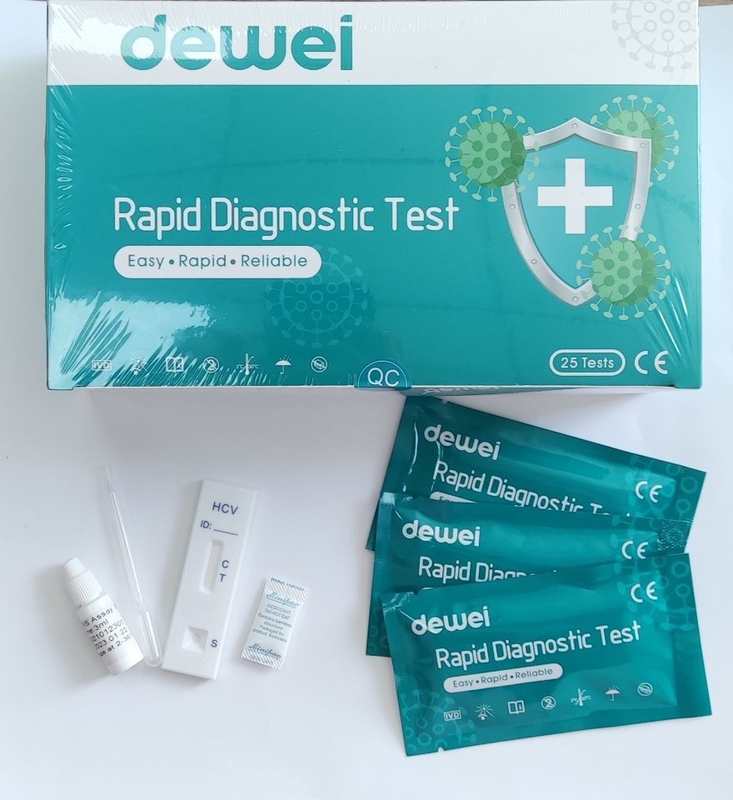
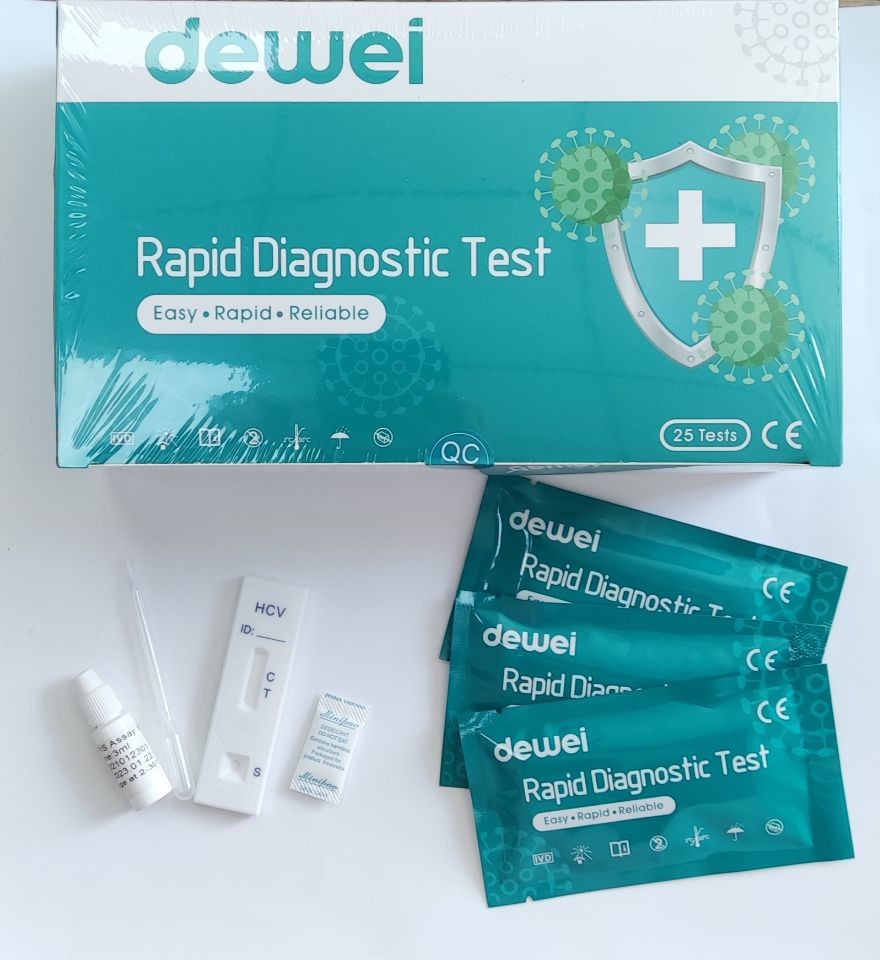
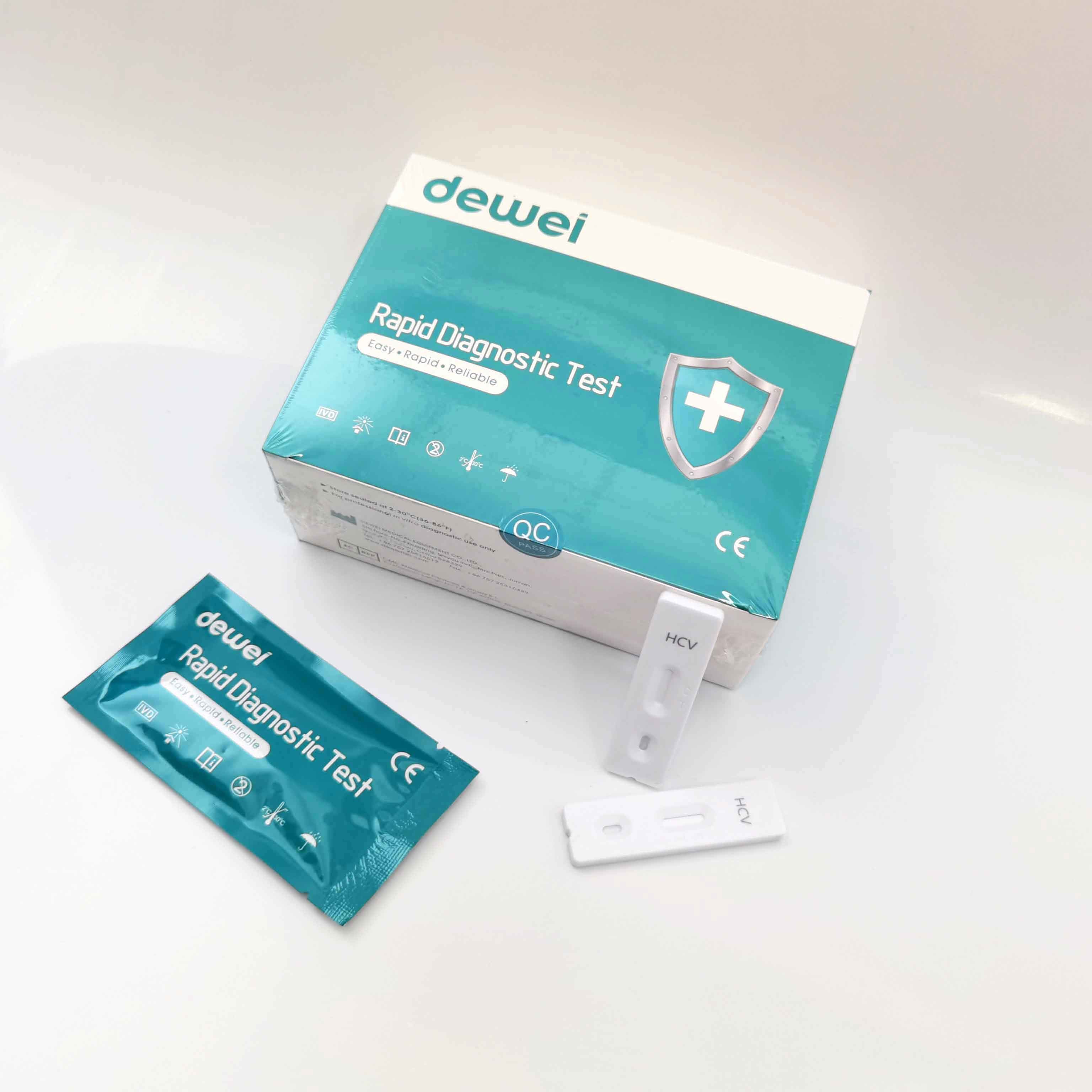
• One pouch containing a reaction test Cassette with desiccant.
• Disposable pipettes.
• Buffer.
• Instructions for use.
STORAGE AND STABILITY
• Store at 39~ 86 º F (4 ~ 30 º C) in the sealed pouch for 18 months.
PRECAUTIONS
• For in vitro diagnostic use only.
• Do not use after expiration date.
• The test Cassette should remain in the sealed pouch until use.
• The used test Cassette should be discarded according to local regulations.
DIRECTION OF USE
Bring tests, specimens, and/or controls to room temperature (15-30°C) before use.
1. Remove the test from its sealed pouch, and place it on a clean, level surface. Label the device with patient or control identification. For best results, the assay should be performed within one hour.
2. Using the provided disposable pipette, transfer 1 drop of serum/plasma specimen (approximately 25 µL) to the specimen well (S) of the device, then add 1 drop of buffer and start the timer.
Avoid trapping air bubbles in the specimen well (S), and do not add any solution to the result area.
As the test begins to work, color will migrate across the membrane.
3. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
INTERPRETATION
POSITIVE: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
NEGATIVE: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T).
INVALID: Control band fails to appear. Results from any test which has not produced a control band at the specified read time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.



 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!