H. pylori Antibody Rapid Diagnostic Test Kit Whole Blood Serum Plasma One Step
INTENDED USE
The H. pylori Antibody Rapid Test (Whole Blood/Serum/Plasma) is a rapid visual immunoassay for the qualitative presumptive detection of specific IgM and IgG antibodies to Helicobacter pylori in human whole blood, serum, or plasma specimens. This kit is intended for use as an aid in the diagnosis of H. pylori infection.
INTRODUCTION
Gastritis and peptic ulcers are among the most common human diseases. Since the discovery of H. pylori (Warren & Marshall, 1983), many reports have suggested that this organism is one of the major causes of ulcer diseases (Anderson & Nielsen, 1983; Hunt & Mohamed, 1995; Lambert et al, 1995). Although the exact role of H. pylori is not yet fully understood, eradication of H. pylori has been associated with the elimination of ulcer diseases. The human serological responses to infection with H. pylori have been demonstrated (Varia & Holton, 1989; Evans et al, 1989). The detection of IgG antibodies specific to H. pylori has been shown to be an accurate method for detecting H. pylori infection in symptomatic patients. H. pylori may colonize some asymptomatic people. A serological test may be used either as an adjunct to endoscopy or as an alternative measure in symptomatic patients.
PRINCIPLE
The H. pylori Antibody Rapid Test (Whole Blood/Serum/Plasma) detects IgM and IgG antibodies specific to Helicobacter pylori through visual interpretation of color development on the internal strip. H. pylori antigens are immobilized on the test region of the membrane. During testing, the specimen reacts with H. pylori antigen conjugated to colored particles and precoated onto the sample pad of the test. The mixture then migrates through the membrane by capillary action, and interacts with reagents on the membrane. If there are sufficient antibodies to Helicobacter pylori in the specimen, a colored band will form at the test region of the membrane. The presence of this colored band indicates a positive result, while its absence indicates a negative result. The appearance of a colored band at the control region serves as a procedural control, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
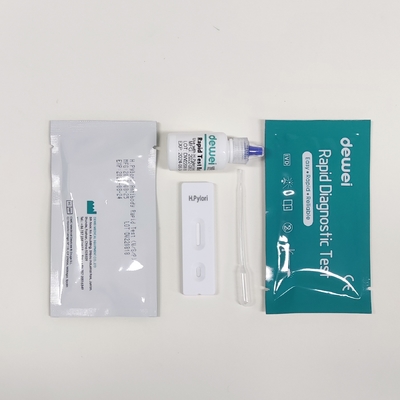
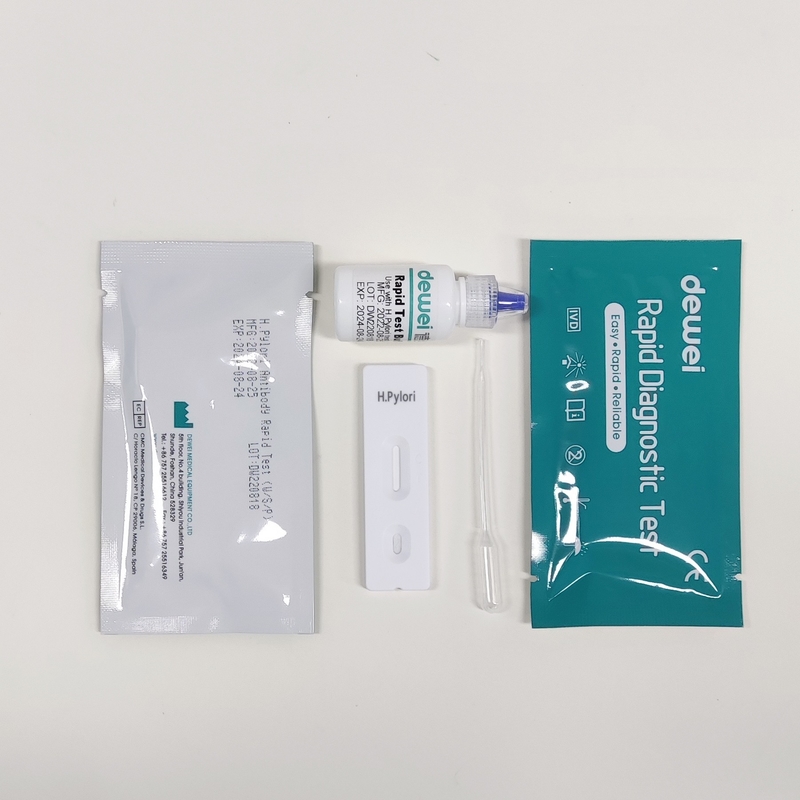
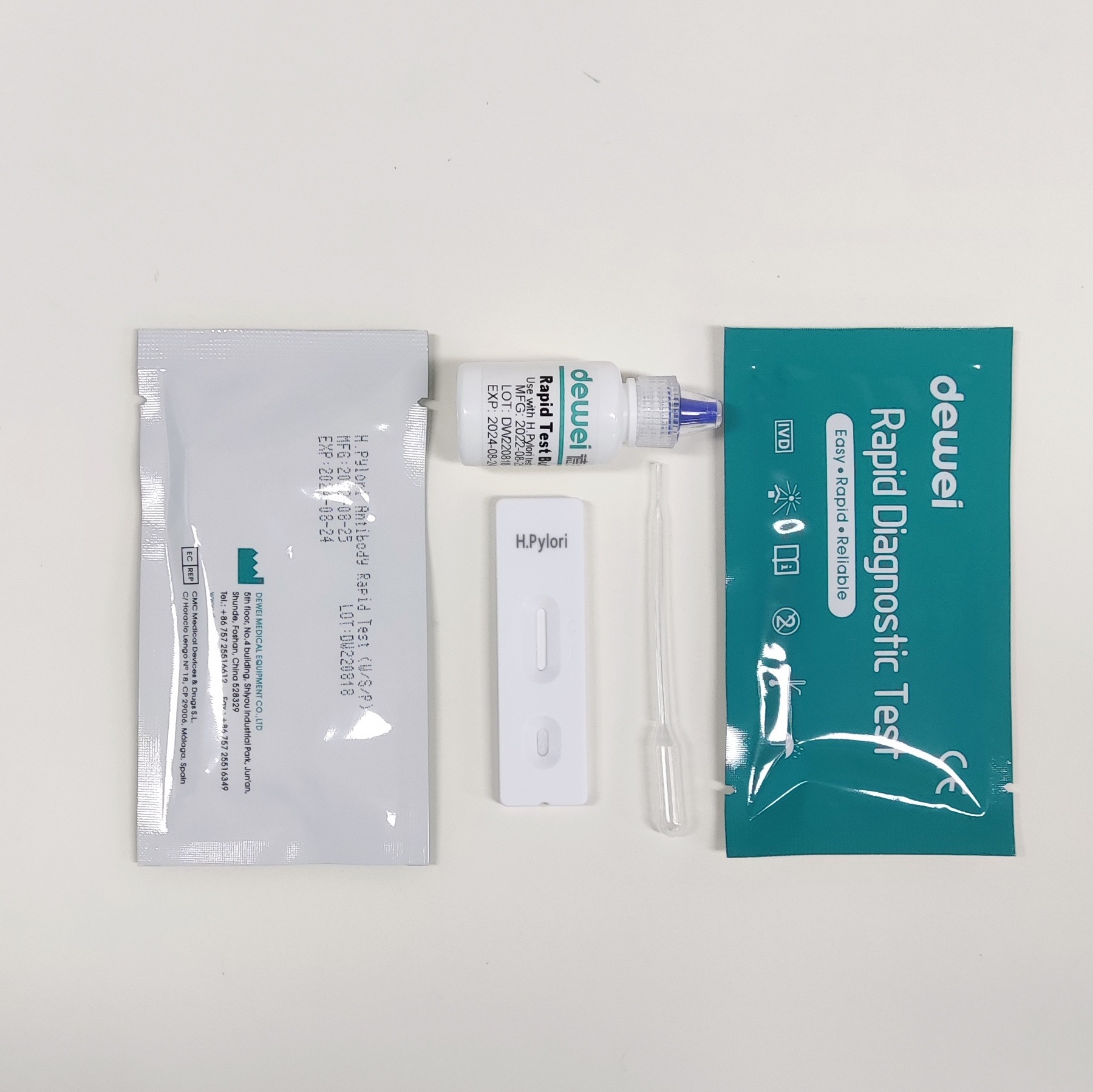
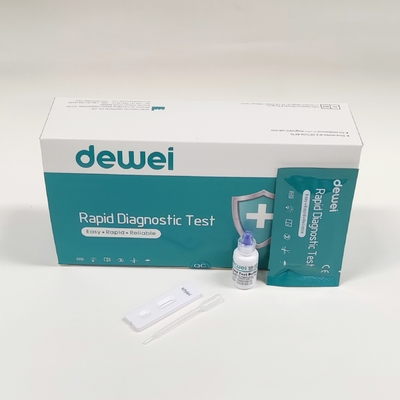
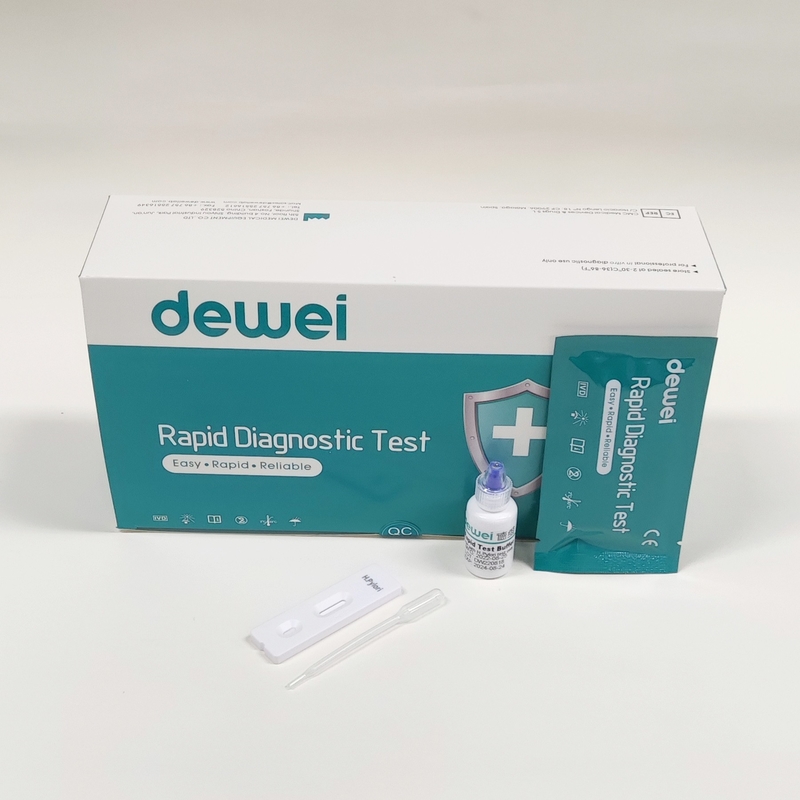
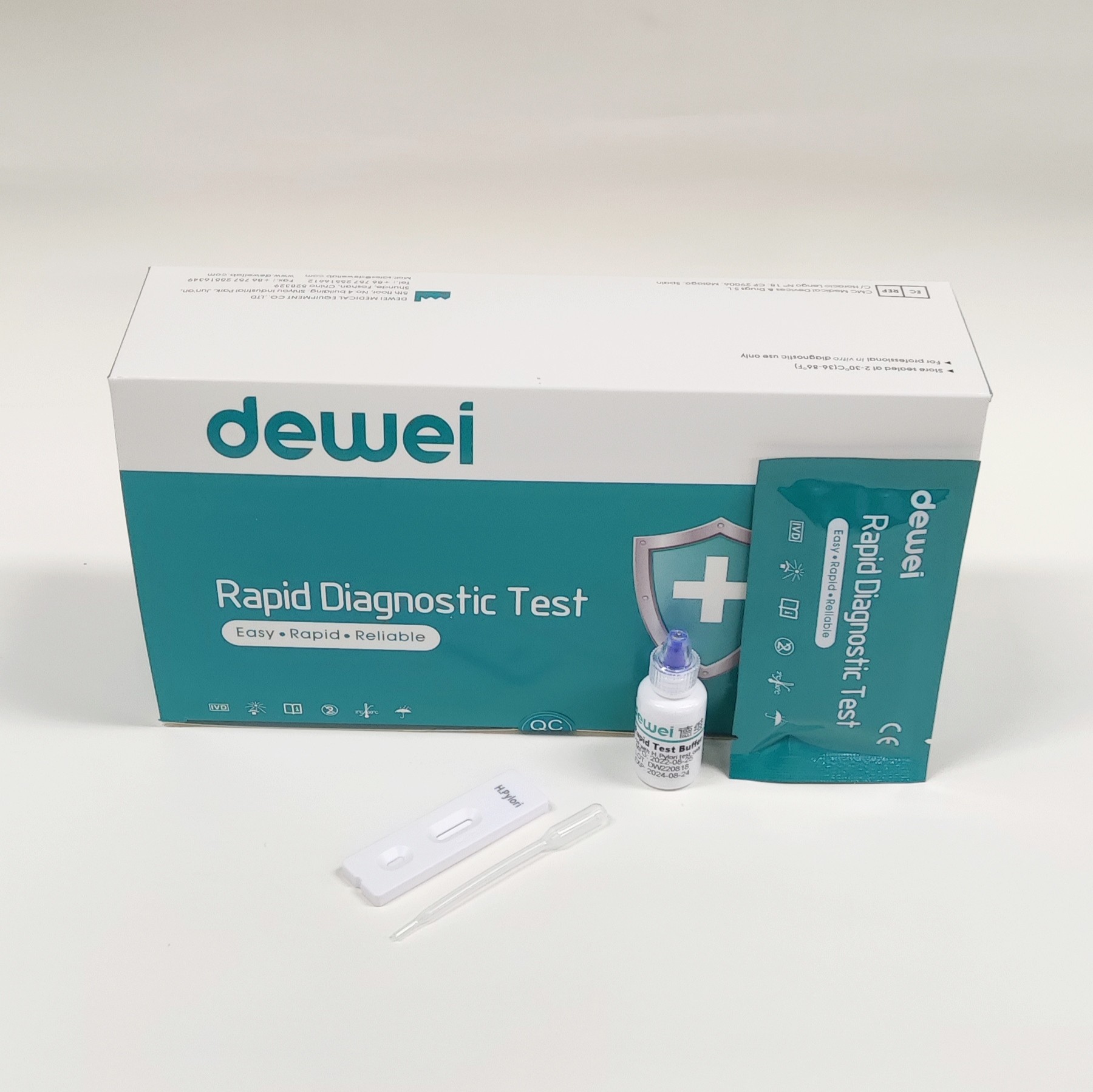
MAIN CONTENTS
Rapid Test Cassette in Pouch
Buffer
Disposable dropper
Package insert
PRECAUTIONS
- For professional in vitro diagnostic use only.
- Do not use after the expiration date indicated on the package. Do not use the test if the foil pouch is damaged. Do not reuse tests.
- This kit contains products of animal origin. Certified knowledge of the origin and/or sanitary state of the animals does not completely guarantee the absence of transmissible pathogenic agents. It is therefore, recommended that these products be treated as potentially infectious, and handled by observing usual safety precautions (e.g., do not ingest or inhale).
- Avoid cross-contamination of specimens by using a new specimen collection container for each specimen obtained.
- Read the entire procedure carefully prior to testing.
- Do not eat, drink or smoke in the area where the specimens and kits are handled. Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout the procedure and follow standard procedures for the proper disposal of specimens. Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
- Humidity and temperature can adversely affect results.
- Used testing materials should be discarded according to local regulations.
SRORAGE
Store as packaged in the sealed pouch at room temperature or refrigerated (2-30°C).
The test is stable through the expiration date printed on the sealed pouch.
The test must remain in the sealed pouch until use.
DO NOT FREEZE.
Do not use beyond the expiration date.
OPERATION PROCEDURE
Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.
1. Remove the test from its sealed pouch, and place it on a clean, level surface. Label the device with patient or control identification. For best results the assay should be performed within one hour.
2. Transfer 1 drop of whole blood or serum or plasma (approximately 25 µL) to the specimen well (S) of the device with the provided disposable pipette, then add 3 drops of buffer and start the timer.
OR
Allow 1 drop of whole blood or fingerstick whole blood specimen (approximately 25 µL) to the specimen well (S) of the device, then add 3 drops of buffer and start the timer.
Avoid trapping air bubbles in the specimen well (S), and do not add any solution to the result area.
As the test begins to work, color will migrate across the result area in the center of the device.
3. Wait for the colored band(s) to appear. The result should be read at 5 minutes. Do not interpret the result after 10 minutes.
INTERPRETATION
POSITIVE: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
NEGATIVE: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T).
INVALID: Control band fails to appear. Results from any test which has not produced a control band at the specified read time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
Note:
- The intensity of color in the test region (T) may vary depending on the concentration of analytes present in the specimen. Therefore, any shade of color in the test region should be considered positive. Note that this is a qualitative test only, and cannot determine the concentration of analytes in the specimen.
- Insufficient specimen volume, incorrect operating procedure or expired tests are the most likely reasons for control band failure.
FRQ:
1) What is the cause of H pylori infection?
H. pylori bacteria are usually passed from person to person through direct contact with saliva, vomit or stool. H. pylori may also be spread through contaminated food or water. The exact way H. pylori bacteria causes gastritis or a peptic ulcer in some people is still unknown.
2) What are the signs and symptoms of H. pylori?
H. pylori is a bacteria that can cause peptic ulcer disease and gastritis. It mostly occurs in children. Only 20% of those infected have symptoms. Symptoms include dull or burning stomach pain, unplanned weight loss and bloody vomit.
3) Can H. pylori be cured completely?
In some cases, H pylori can't be cured with any therapy, though the symptoms may be able to be reduced. If cured, reinfection may occur in areas where sanitary conditions are poor.
4) How serious is H pylori infection?
It can damage the tissue in your stomach and the first part of your small intestine (the duodenum). This can cause pain and inflammation. In some cases, it can also cause painful sores called peptic ulcers in your upper digestive tract. H. pylori is common.
5) What do bowel movements look like with H. pylori?
pylori infection, you might have blood in your stool. In most cases, the blood appears very dark — almost black. Your stools might have a tarry appearance or consistency. If you have bloody stools, you should call our office right away so we can get any bleeding under control.
6) What not to eat when you have H. pylori?
Citrus fruits such as lemons and pineapple can increase stomach acid and cause pain and heartburn. Spicy foods. Spicy or acidic foods like garlic, mustard and pepper can all have a negative effect on people with H.
7) What is the fastest way to cure H. pylori?
Ulcers caused by H. pylori are usually treated with a combination of antibiotics and a proton pump inhibitor (PPI). Triple therapy: Therapies that combine PPIs with two antibiotics remain the first-line options for treating H. pylori.
8) What is the first stage of H. pylori?
Although a staging system for the H pylori infection does not exist, some steps in the disease process are well described. The first step is chronic gastritis, followed after a time by the second step, atrophic gastritis. The third step is intestinal metaplasia, which may evolve into dysplasia.
9) What happens if you test positive for H. pylori?
A positive test result means that you have an H. pylori infection. Your provider will usually prescribe one or more antibiotics to treat the infection. You will usually take other medicines to relieve your symptoms and help heal your stomach.
10) How contagious is H. pylori?
H. pylori is commonly transmitted person-to-person by saliva. The bacteria can also be spread by fecal contamination of food or water. In developing countries, a combination of untreated water, crowded conditions, and poor hygiene contributes to higher H.
11) Can your body get rid of H. pylori by itself?
The almost invincible human immune system fails to eliminate H. pylori because of successful immune evasion strategies and also because of the complex intrinsic genetic variability adopted by this bacterium.
12) Which probiotic kills H. pylori?
pylori treatment reveals that single-strained probiotics, particularly those containing Lactobacillus spp., Bifidobacterium spp., and Saccharomyces spp. are more commonly used. These probiotics have shown promise in increasing the success rate of H. pylori eradication and in mitigating side effects.
13) Can stress cause H. pylori?
Certain health and lifestyle factors increase the risk of damage to the stomach and intestinal lining. These factors make it more likely that a person will develop an ulcer, including a stress-related ulcer: H. pylori infection.
14) What is the natural killer of H. pylori?
In human studies , daily consumption of broccoli sprouts reduced H. pylori colonization and gastric inflammation, with some cases resulting in complete eradication of the bacteria.
For details, please contact Dewei staff for Instruction Manual!




 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!