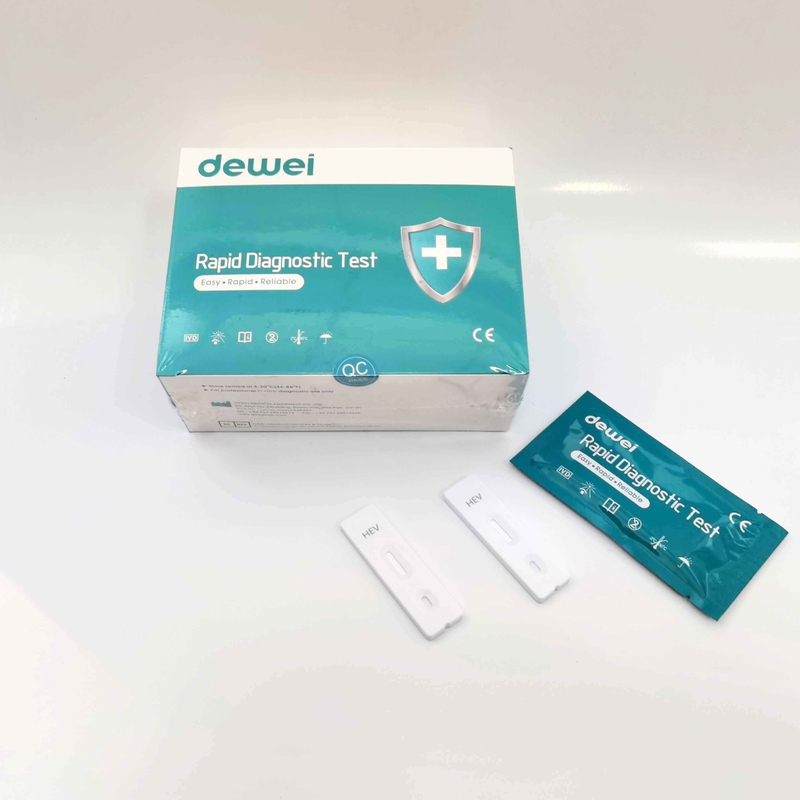
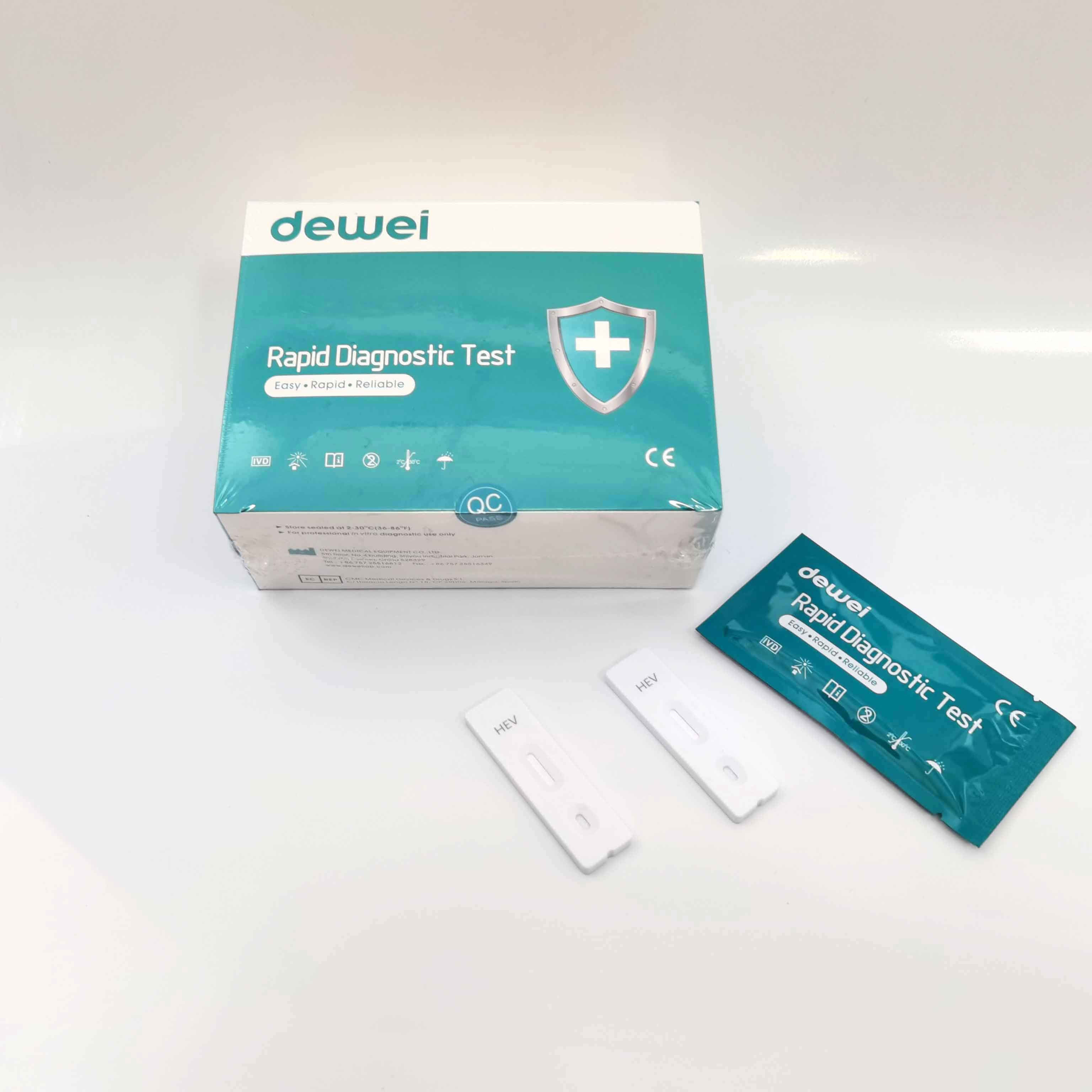
HEV IgM Rapid Diagnostic Kit 10 Mins Hepatitis E Virus Test Device Strip
【INTRODUCTION OF HEV】
Hepatitis E is a liver infection caused by the hepatitis E virus (HEV). HEV is found in the stool of an infected person. It is spread when someone unknowingly ingests the virus – even in microscopic amounts. In developing countries, people most often get hepatitis E from drinking water contaminated by feces from people who are infected with the virus.
【INTENDED USE OF HEV RAPID TEST】
HEV IgM Rapid Test is a lateral flow immunoassay for the qualitative detection of IgM antibodies to hepatitis E virus (HEV) in human serum, plasma or whole blood. It is intended to be used by professionals as a preliminary test result to aid in the diagnosis of infection with HEV.
【PRINCIPLE】
The HEV Rapid Test Cassette (Serum/Plasma) is a qualitative membrane-based immunoassay for the detection of HEV antibodies in serum or plasma. This test consists of two components, an IgG component and an IgM component. In the IgG component, anti-human IgG is coated in IgG test line region. During testing, the specimen reacts with HEV antigen-coated particles in the test cassette. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with the anti-human IgG in IgG test line region. If the specimen contains IgG antibodies to HEV, a colored line will appear in IgG test line region. In the IgM component, anti-human IgM is coated in IgM test line region. During testing, the specimen reacts with anti-human IgM. HEV IgM antibodies, if present in the specimen, reacts with the anti-human IgM and the HEV antigen-coated particles in the test cassette, and this complex is captured by the anti-human IgM, forming a colored line in IgM test line region. Therefore, if the specimen contains HEV IgG antibodies, a colored line will appear in IgG test line region. If the specimen contains HEV IgM antibodies, a colored line will appear in IgM test line region. If the specimen does not contain HEV antibodies, no colored line will appear in either of the test line regions, indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
【DIAGNOSTIC PRINCIPLE】
Chromatographic Immunoassay
【MAIN CONTENTS】
Individually sealed foil pouches containing a test cassette with desiccant
Disposable pipettes
Buffer
Insert
【RESULT INTERPRETATION】
NEGATIVE RESULT:
If only the C line develops, the test indicates that no detectable anti-HEV IgM is present in the specimen. The result is negative or non-reactive.
POSITIVE RESULT:
If both the C and the T lines develop, the test indicates the presence of detectable anti-HEV IgM in the specimen. The result is positive or reactive.
INVALID:
If no C line develops, the assay is invalid regardless of any color development on the T line as indicated below. Repeat the assay with a new device.
【STORAGE AND STABILITY】
The kit should be stored at 2-30°C until the expiry date is printed on the sealed pouch.
The test must remain in the sealed pouch until use.
Keep away from direct sunlight, moisture and heat. Do not freeze.
Care should be taken to protect the components of the kit from contamination. Do not use it if there is evidence of microbial contamination or precipitation. Biological contamination of dispensing equipment, containers, or reagents can lead to false results.


 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!