【INTRODUCTION】
Treponema Pallidum (TP), a spirochete bacterium with an outer envelope and a cytoplasmic membrane, is the causative agent of the venereal disease syphilis.
Although syphilis rates are declining in the United States after an epidemic between 1986 and 1990, the incidence of syphilis in Europe has increased since 1992, especially in the countries of the Russia Federation, where peaks of 263 cases per100,000 have been reported. In addition, the positive rate of serological test results for syphilis in HIV-infected individuals has been rising recently.
The serological detection of specific antibodies to TP has been long recognized in the diagnosis of syphilis since the natural course of the infection is characterized by periods without clinical manifestations. The antibody response to TP can be detected within 4 to 7 days after the syphilis chancre appears, allowing early detection and diagnosis of syphilis infection.
A variety of antigens have been used in syphilis serological tests, such as Rapid Plasma Cardiolipin (RPR) or VDRL antigen, TP extracts derived from in vitro culture or inoculated rabbit testes. However, RPR and VDRL antigens are not treponemal specific, and whole TP extracts are not reproducible and contain a certain amount of contaminating materials such as flagella, which may lead to a nonspecific reaction in assays of test serum.
【PRINCIPLE】
The Syphilis Rapid Test detects antibodies to Treponema Pallidum (TP) through visual interpretation of color development on the internal strip. Specific recombinant TP antigens are immobilized on the test region of the membrane. During testing, the specimen reacts with recombinant TP-specific antigen conjugated to colored particles and precoated onto the sample pad of the test. The mixture then migrates through the membrane by capillary action and interacts with reagents on the membrane. If there are sufficient antibodies to Treponema Pallidum (TP) in the specimen, a colored band will form at the test region of the membrane. The presence of this colored band indicates a positive result, while its absence indicates a negative result. The appearance of a colored band at the control region serves as a procedural control, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
【MAIN CONTENTS】
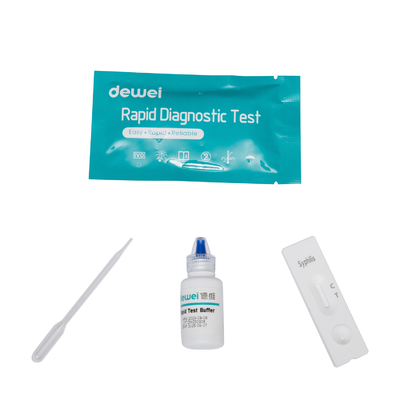
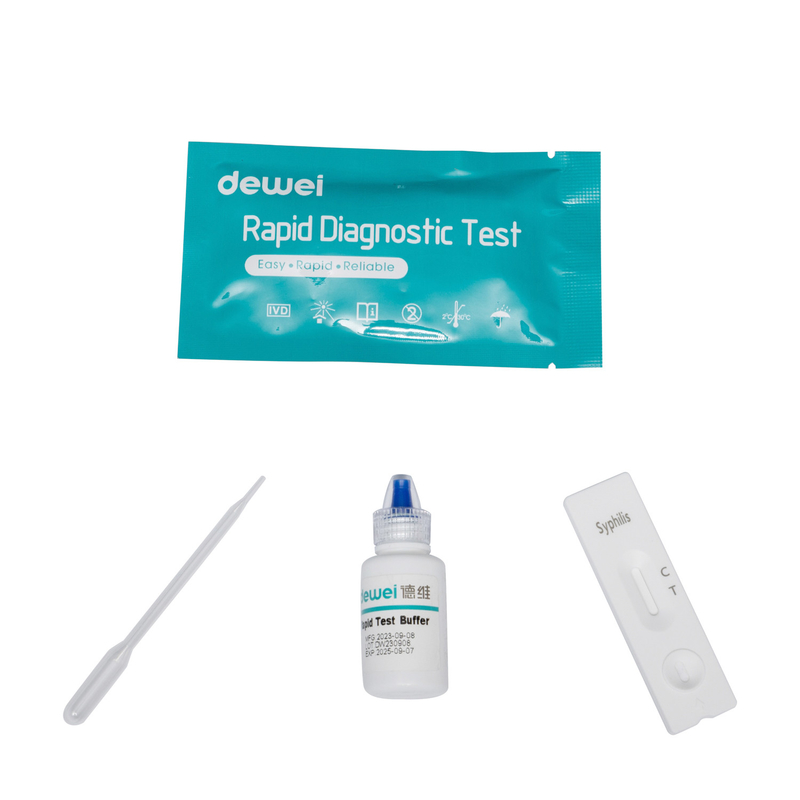
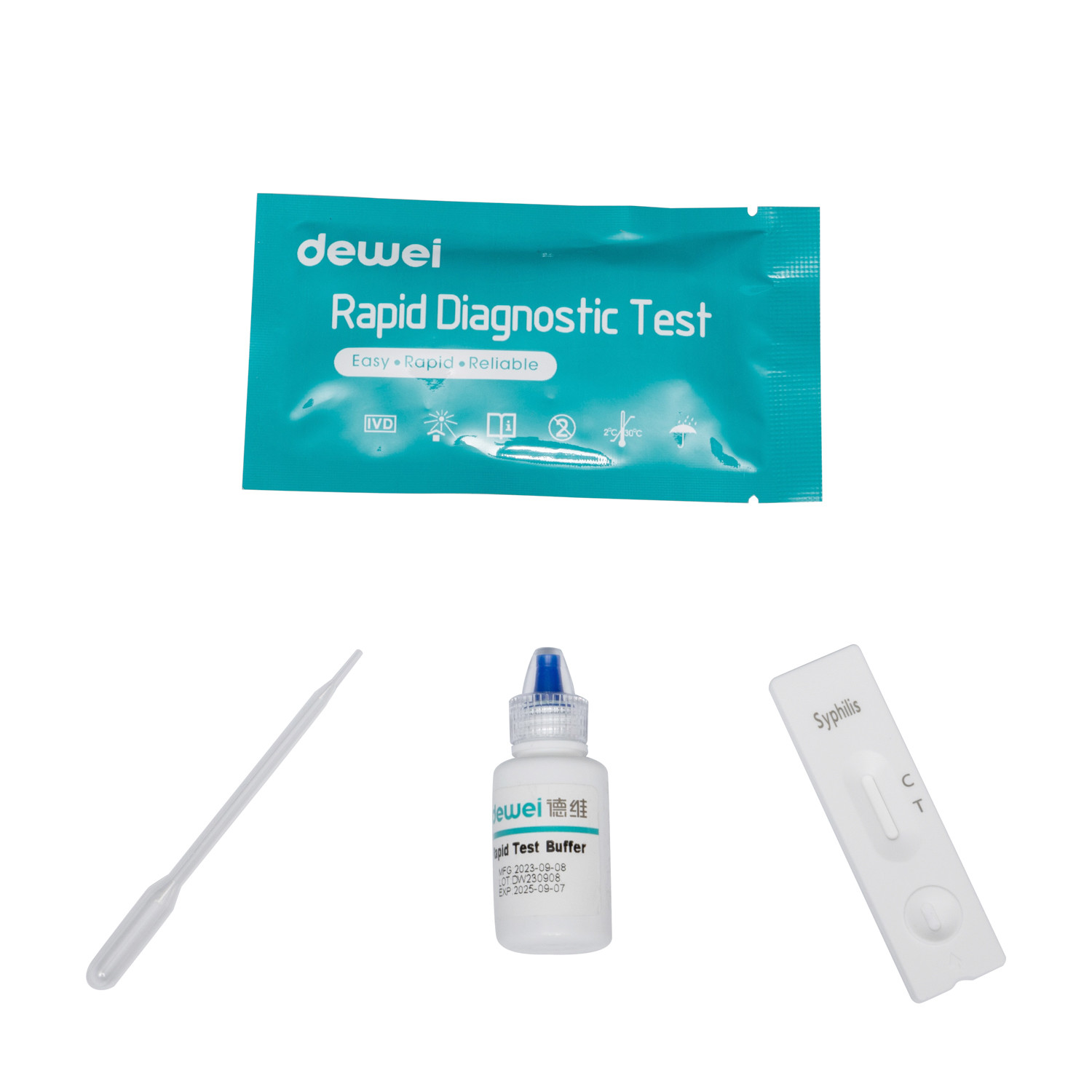
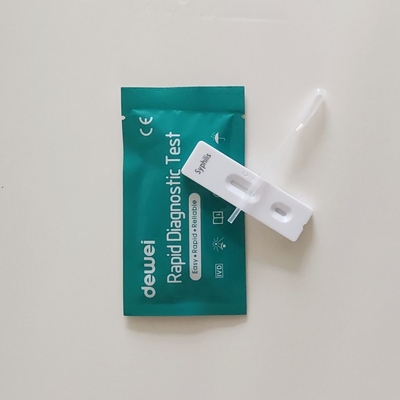
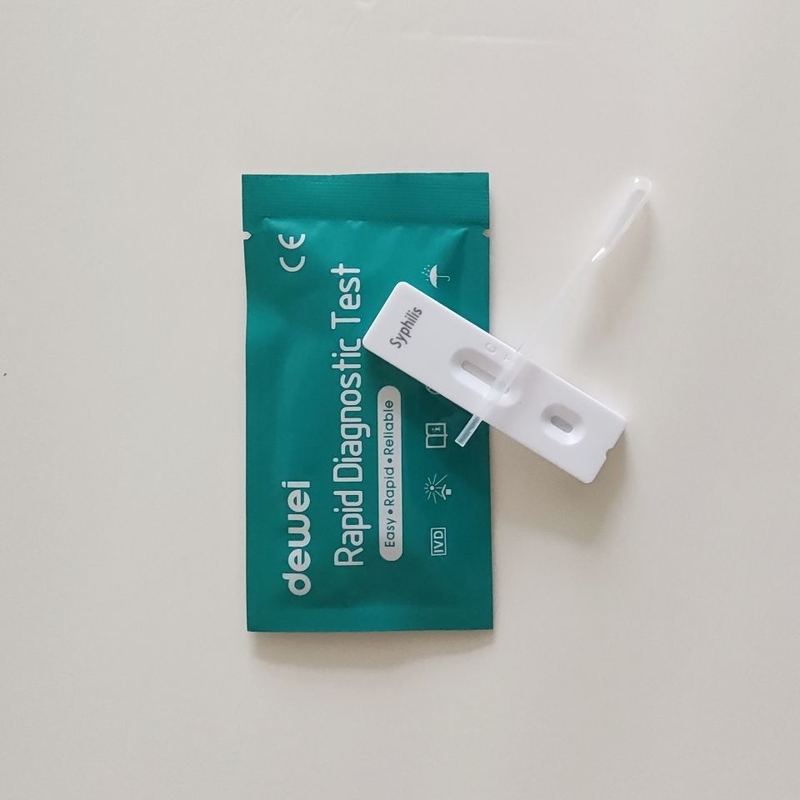
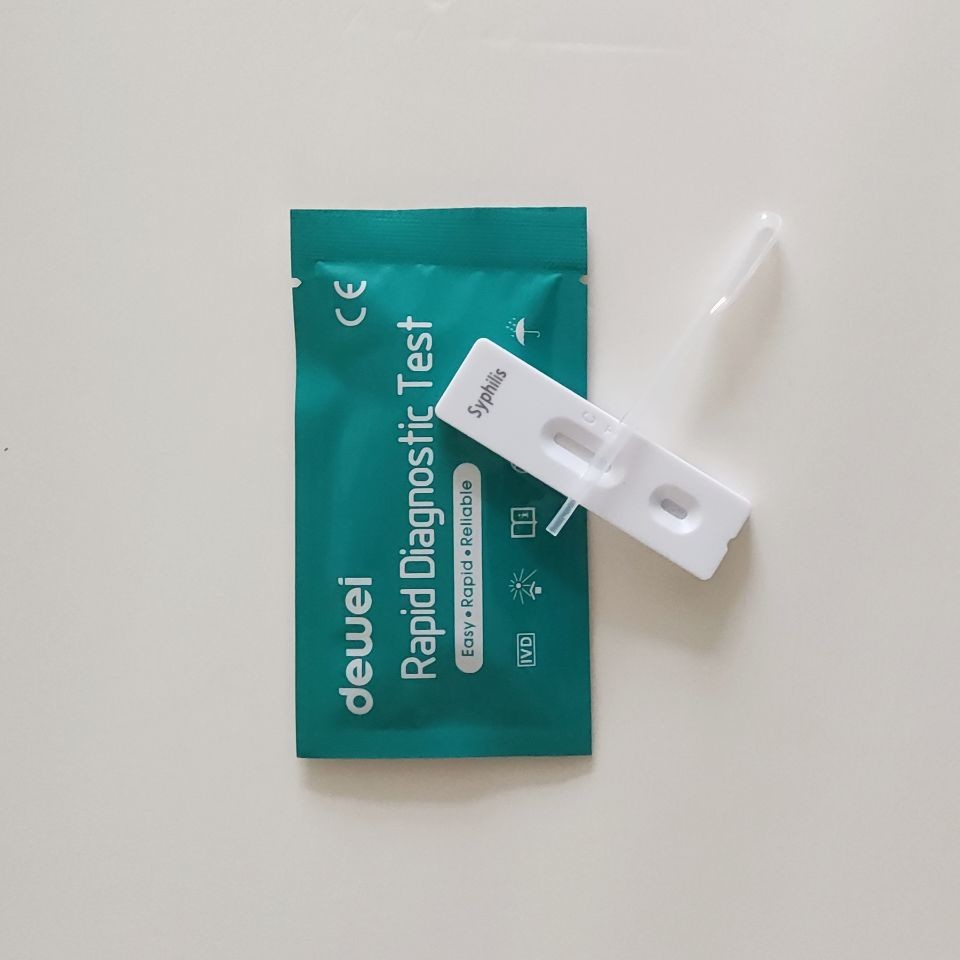
• Rapid test cassette with desiccant
• Buffer
• Disposable pipettes
• Package insert
【PRECAUTIONS】
• For professional in vitro diagnostic use only.
• Do not use after the expiration date indicated on the package. Do not use the test if the foil pouch is damaged. Do not reuse tests.
• This kit contains products of animal origin. Certified knowledge of the origin and/or sanitary state of the animals does not completely guarantee the absence of transmissible pathogenic agents. It is therefore, recommended that these products be treated as potentially infectious, and handled by observing usual safety precautions (e.g., do not ingest or inhale).
• Avoid cross-contamination of specimens by using a new specimen collection container for each specimen obtained.
• Read the entire procedure carefully prior to testing.
• Do not eat, drink or smoke in the area where the specimens and kits are handled. Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout the procedure and follow standard procedures for the proper disposal of specimens. Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
• Humidity and temperature can adversely affect results.
• Used testing materials should be discarded according to local regulations.
【STORAGE AND STABILITY】
• Store at 2 ~ 30 º C in the sealed pouch for 24 months.
• Keep away from direct sunlight, moisture and heat.
• DO NOT FREEZE.
【SAMPLE COLLECTION】
• The Syphilis Rapid Test is intended for use with human Whole Blood, serum or plasma specimens only.
• Only clear, non-hemolyzed specimens are recommended for use with this test. Serum or plasma should be separated as soon as possible to avoid hemolysis.
• Perform testing immediately after specimen collection. Do not leave specimens at room temperature for prolonged periods. Serum and plasma specimens may be stored at 2-8°C for up to 3 days. For long term storage, specimens should be kept below -20°C. Whole blood collected by venipuncture should be stored at 2-8°C if the test is to be run within 2 days of collection. Do not freeze whole blood specimens. Whole blood collected by fingerstick should be tested immediately.
• Containers containing anticoagulants such as EDTA, citrate, or heparin should be used for whole blood storage.
• Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Avoid repeated freezing and thawing of specimens.
• If specimens are to be shipped, pack them in compliance with all applicable regulations for transportation of etiological agents.
• Icteric, lipemic, hemolysed, heat treated and contaminated specimens may cause erroneous results.
【DIRECTION OF USE】
Bring tests, specimens, buffer and/or controls to room temperature (15- 30°C) before use.
1.Remove the test from its sealed pouch, and place it on a clean, level surface. Label the test with patient or control identification. For best results, the assay should be performed within one hour.
2.Using the provided disposable pipette, transfer 2 drops of serum/Plasma to the specimen well (S) of the cassette with the provided disposable pipette, then start the timer.
OR
Transfer 1 drop of whole blood specimen to the specimen well (S) of the device cassette with the provided disposable pipette, then add 1 drop of buffer and start the timer.
Avoid trapping air bubbles in the specimen well (S), and do not add any solution to the result area.
As the test begins to work, color will migrate across the membrane.
3. Wait for the colored band(s) to appear. The result should be read at 15 minutes.
Do not interpret the result after 20 minutes.
【INTERPRETATION OF RESULTS】
POSITIVE: The presence of two lines as control line (C) and test line (T) within the result window indicates a positive result.
NEGATIVE: The presence of only control line (C) within the result window indicates a negative result.
INVALID: If the control line (C) is not visible within the result window after performing the test, the result is considered invalid. Some causes of invalid results are because of not following the directions correctly or the test may have deteriorated beyond the expiration date. It is recommended that the specimen be re-tested using a new test.
For further operation or performance details, please refer to final instruction manual.

 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!